南湖新闻网讯(通讯员 贾崇昊)2030年前实现碳达峰、2060年前实现碳中和的双碳目标是我国重大的战略目标之一。为促进双碳目标的实现,我校生物-地质矿化研究团队近年来聚焦于土壤矿物对有机质的固定过程展开了系列研究。目前最新研究成果陆续发表在环境领域期刊Environmental Science & Technology、Environmental Science : Nano上,并在在环境领域综述期刊Reviews in Environmental Science & Bio-Technology上发表了题为“Retention of soil organic matter by occlusion within soil minerals”的综述论文。
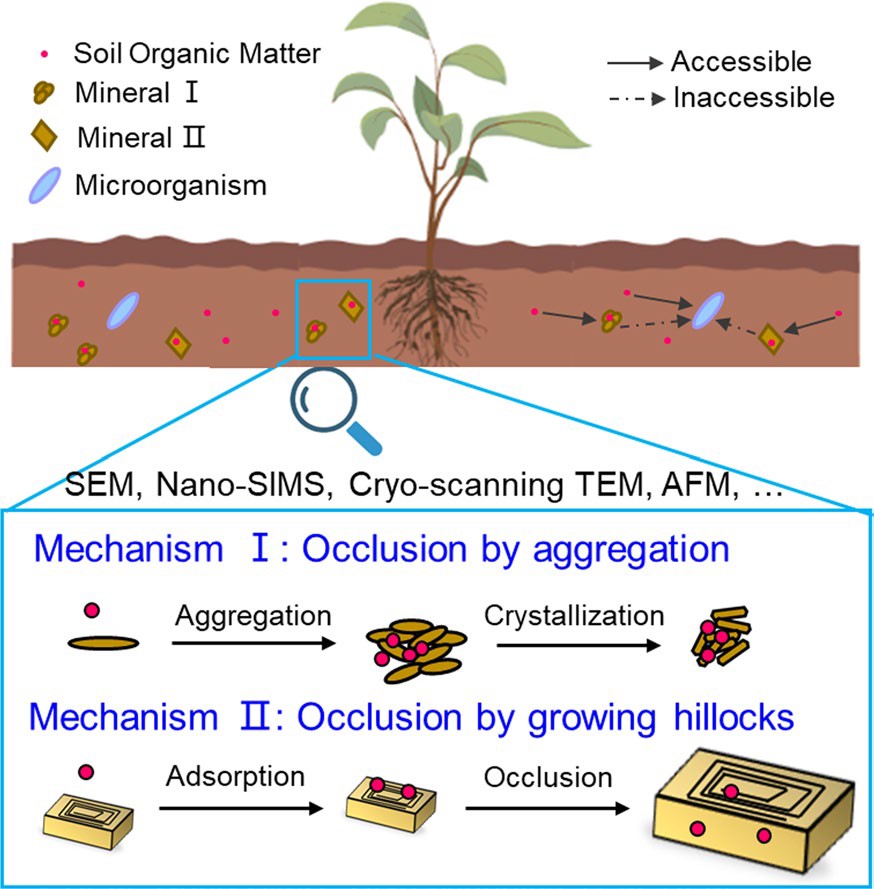
图1 矿物通过颗粒黏附和螺旋生长方式包埋土壤有机质的示意图。
土壤是重要的有机碳库,其土壤有机质(SOM)含量可以达到其它生态系统(如大气、海洋)至少三倍以上,这些土壤有机质稳定性对于土壤肥力、水质、食品安全具有重要意义,且有机质可以被多种生物和非生物因素分解为二氧化碳、甲烷等温室气体,从而显著影响全球气候变化。传统的研究主要关注于有机质在矿物表面的吸附解吸等界面过程,对于封存的动力学过程和微观机制却鲜有研究。本研究通过多种微米/纳米尺度的技术表征,发现土壤矿物可通过与有机物聚合以及矿物螺旋生长方式对有机质进行封存固定(图1)。
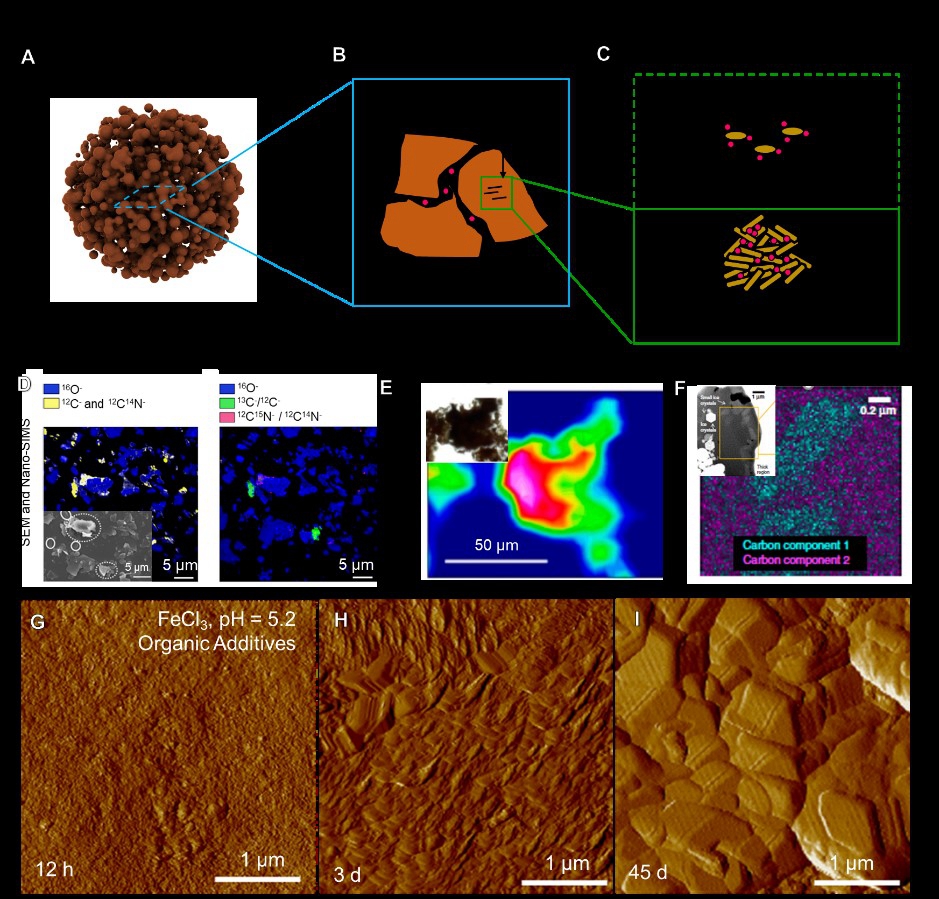
图2: (A-C) 颗粒黏附方式对土壤有机质包埋的示意图。(D-F) 扫描电子显微镜、纳米二次离子质谱、透射电子显微镜观察有机-矿物团聚体对有机质的包埋。(G-I)原子力显微镜原位观察铁氧化物对土壤有机组分的包埋过程。
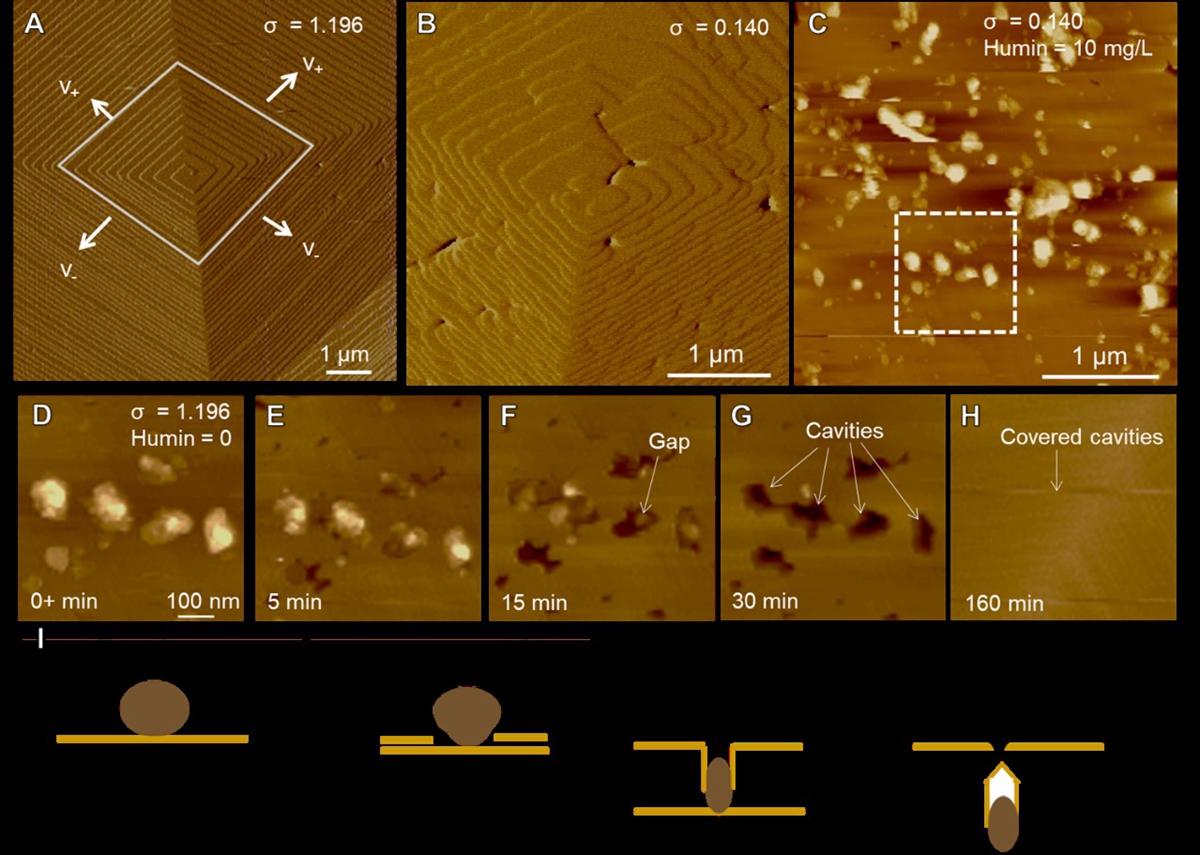
图3:方解石对有机质的吸附和包埋的动力学过程。(A)方解石在碳酸钙过饱和溶液中(通过螺旋生长方式进行生长。(B)在近平衡的碳酸钙过饱和溶液中,台阶的前进速度为0。(C)通入有机质的碳酸钙过饱和溶液后,有机质可以吸附到晶体表面。(D-H)重新通入σ = 1.196的碳酸钙过饱和溶液后,吸附的有机质颗粒伴随着台阶的前进持续被包埋进入晶体中。(I)方解石包埋有机质的示意图。
矿物-有机聚合固定有机质过程广泛存在于金属氧化物或氢氧化物以及层状硅酸盐等粘土矿物中(图2)。在该过程中有机组分首先通过静电作用、范德华力、氢键等机制吸附到矿物的表面,之后有机-矿物的颗粒可以进一步聚合,并伴随发生矿物的相转变以及结晶过程对有机质进行封存。除了颗粒聚合的方式外,矿物还可以通过螺旋生长的方式包裹有机质,吸附的有机质可以通过以下三个步骤被前进的晶面台阶逐渐包埋封存进入矿物内部(图3):前进的台阶开始覆盖和包埋吸附的有机质颗粒;吸附的颗粒受到台阶的压缩,在这个过程中会有缺陷或者空洞的产生;空洞被彻底封闭,有机质最终被矿物内部封存。以上过程受到矿物的性质(形貌、结晶度、晶面性质)、有机质性质(表面功能基团、带电性、分子量)以及土壤溶液条件的共同调控。
上述研究得到国家自然科学基金,国家重点研发计划和校自主科技创新基金的支持。系列论文第一作者为博士研究生迟家霖,通讯作者为王荔军教授和张文君副教授。
审核人:张文君
【英文摘要1】
Organo–clay complexes could be adsorbed and subsequently occluded into soil mineral matrices under local supersaturated solution conditions, leading to inaccessibility of microorganisms and their extracellular enzymes, which plays an important contribution to stabilization of soil organic matter (SOM) and affects their biogeochemical cycle. However, the underlying molecular mechanisms remain poorly understood. Here we apply Raman spectroscopy to analyze the LAPONITE®-sugar (monosaccharide glucose (Glu) and 5/20 kDa dextran (Dex-5/20) polysaccharides) interactions and use in situ atomic force microscopy (AFM) to observe their occlusion processes within calcite. As shown by Raman spectra, the LAPONITE®-sugar complexes form with the mix of sugars and LAPONITE®, and the degree of elution is mediated by the molecular weight of sugars and more Glu would be eluted compared with Dex-5 and Dex-20. Then the LAPONITE®-sugar complexes could be occluded within calcite observed by AFM, and the occlusion of the LAPONITE®-sugar complexes within calcite hillocks are influenced by molecular weight with the trend of Dex-20 > Dex-5 > Glu. The binding force between sugars and calcite (10 4) surfaces are measured by AFM-based dynamic force spectroscopy (DFS) to record the molecular-scale interactions, and high-molecular weight sugar such as Dex-20 exhibits strongest binds with calcite surfaces as shown by DFS data. These in situ nanoscale observations and single-molecule determinations in a model system may provide insights into the clay-SOM-calcite fixation mechanisms by sugar in alkaline soils, with potential implications for global carbon cycling.
【英文摘要2】
Nanoplastics are widely distributed in crop soils and can interact with other exposed organic contaminants such as pesticides, leading to enhanced toxicity to plants and soil-beneficial microorganisms. These combined organic pollutants can also interact physiochemically with mineral matrices, becoming selectively preserved and occluded. Inclusion organics within growing minerals and the pore spaces of mineral aggregates are potentially inaccessible to plant root cells and soil microorganisms due to the limitation of their movement, but the microscopic mechanisms that control occlusion processes in the presence of nanoplastics mixed with pesticides remain poorly understood. Here, we use time-resolved atomic force microscopy (AFM) to observe how model soil minerals interact in situ with different functional groups of polystyrene (PSFG) mixed with glyphosate (PMG). Our results show that the PSFG–PMG complexes are occluded within calcite and iron hydroxide particles through hillock growth and aggregation, respectively. The free energies of binding between the functional groups of polystyrene and calcite surfaces measured using AFM-based dynamic force spectroscopy in the presence of different concentrations of PMG account for the molecular interactions involved in the occlusion process and the effects of the PMG concentration. These in situ nanoscale observations and molecular-scale energetic measurements in a simple model system may provide insights into the immobilization of both nanoplastics and pesticides by soil minerals, with potential implications relating to multiple pollutant sequestration.
【英文摘要3】
Global soil carbon cycling plays a key role in regulating and stabilizing the earth’s climate change because of soils with amounts of carbon at least three times greater than those of other ecological systems. Soil minerals have also been shown to underlie the persistence of soil organic matter (SOM) through both adsorption and occlusion, but the microscopic mechanisms that control the latter process are poorly understood. Here, using time-resolved in situ atomic force microscopy (AFM) to observe how calcite, a representative mineral in alkaline soils, interacts with humic substances, we show that following adsorption, humic substances are gradually occluded by the advancing steps of spirals on the calcite (1014) face grown in relatively high supersaturated solutions, through the embedment, compression, and closure of humic substance particles into cavities. This occlusion progress is inhibited by phytate at high concentrations (10–100 μM) due to the formation of phytate-Ca precipitates on step edges to prevent the step advancement, whereas phytate at relatively low concentrations (≤1 μM) and oxalate at high concentrations (100 μM) have little effect on this process. These in situ observations may provide new insights into the organo–mineral interaction, resulting in the incorporation of humic substances into minerals with a longer storage time to delay degradation in soils. This will improve our understanding of carbon cycling and immobilization in soil ecological systems.
原文链接:
https://pubs.rsc.org/en/content/articlelanding/2022/en/d1en00902h
https://pubs.rsc.org/en/content/articlelanding/2021/en/d1en00381j

